The mysteries of genetic patenting just got easier with WIPO’s latest ST.26 Sequence Listing Standard. In the realm of patent applications involving nucleotide or amino acid sequences, compliance with international standards is crucial. WIPO’s ST.26 aims to streamline data management, benefiting patent offices, inventors, and applicants alike.
This article delves into the essentials of ST.26, highlighting its innovations, advantages over the previous ST.25 standard, and the critical role sequence listings play in patent applications.
Cracking the Genetic Code: The Significance of Sequence Listings in Biotech Patents
In biotechnology patents, precise documentation of gene mutations, sequencing, and related innovations is vital. Patent offices require structured DNA and protein sequences. This process, known as sequence listing, organizes raw data into a PTO-compliant format.
Genes, composed of amino acid codes, form proteins. Sequence listings identified by “SEQ ID NO.” (e.g., SEQ ID NO: 1), precisely detail these sequences. They’re essential for patent applications disclosing nucleotide and amino acid sequences. When patents disclose sequences, compliance with WIPO’s criteria is a must.
Sequence listings serve dual purposes: compliance and accessibility. They facilitate data searches across patent offices and public databases like EMBL (European Molecular Biology Laboratory), and NCBI (National Center for Biotechnology Information), ensuring smooth information exchange.
Need for Sequence Listing: The Essence of Sequence Listings in Patents”
In patent applications involving biological sequences, a sequence listing is crucial. It compiles this information into a single document, ensuring smooth recording and transmission to searchable databases used by patent offices globally.
Biological sequences, whether synthetic or organic, serve as references for future studies. Standardizing their presentation in searchable databases through a sequence listing simplifies data compilation, offering a comprehensive list of nucleotide and/or amino acid protein sequences.
Previously, compliance with WIPO’s ST.25 standard was vital. Now, the ST.26 standard demands updated adherence, failure of which may lead to rejection.
The new standard aims to harmonize worldwide submission of sequence listings, allowing for a universally accepted application. This initiative enhances accessibility for applicants, the public, and examiners, streamlining sequence searching and electronic data exchange. Understanding previous norms is essential before exploring the new standard.
Decoding the Past: Unraveling the WIPO ST.25 Sequence Listing Standard
ST.25 stands for “WIPO Standard ST.25. The term “ST.25” refers to the international standard adopted in 2009, providing guidelines on presenting nucleotide and amino acid sequences in sequence listings. Serving as the foundation for US sequence regulations (37 CFR 1.821–1.825), ST.25 format is mandatory for applications filed before July 1, 2022.
In an ST.25 sequence listing, fields marked from 110 to 170 constitute the “header” fields, providing essential information applied uniformly to all sequences. These header fields, typically found at the beginning of separate sequence listing documents, help link sequence data to the relevant patent application.
Each sequence in an ST.25 sequence listing is assigned a numbered identifier, starting from “1” and incrementing sequentially. Genetic identification for each sequence is detailed in the <210> and <400> fields. These identifiers, often prefaced with “SEQ ID NO:”, allow easy reference to sequences in patent application descriptions, claims, or drawings.
Until recently, the WIPO ST.25 standard was the norm for sequence listing filings.
Limitations of the ST.25 Sequence Listing Standard and the Need for ST.26
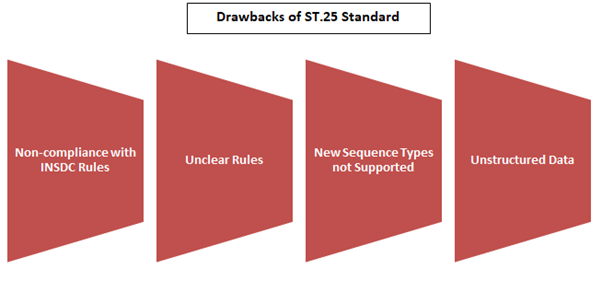
The ST.25 standard, while once prevalent, exhibited several shortcomings that paved the way for the introduction of ST.26:
- Incompatibility with INSDC Specifications: The format prescribed by ST.25 often failed to align with the specifications set by the International Nucleotide Sequence Database Collaboration (INSDC), resulting in data loss upon importation into public databases.
- Lack of Clarity in Regulations: Ambiguities within ST.25 regulations led to varying interpretations by intellectual property offices (IPOs) worldwide, causing inconsistencies in application and enforcement.
- Structural Challenges: Data organization in the ST.25 format lacked structure, posing difficulties for automated validation and efficient data exchange processes.
- Incomplete Coverage: ST.25 failed to encompass modern sequence types like nucleotide analogs, D-amino acids, and branched sequences, rendering them unsearchable in databases. Additionally, the unstructured nature of information hindered verification efforts.
Recognizing these deficiencies, the ST.26 sequence listing standard emerged with the aim of standardizing filing requirements and improving accessibility to nucleotide and amino acid sequences associated with patent applications. The following section delves deeper into the specifics of the ST.26 standard.
ST.26: The Next Era in Sequence Listings
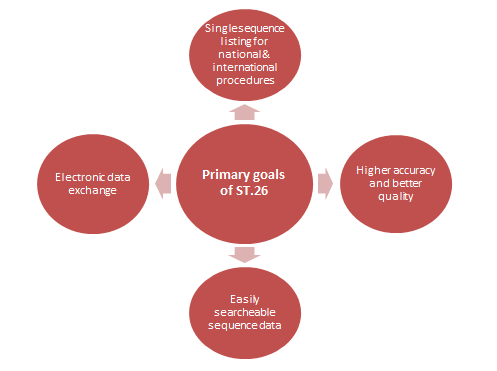
Figure 2: Main Objectives Behind the Introduction of ST.26
Introduced in October 2021 and effective from July 1, 2022, “ST.26” marks a transformative shift in how nucleotide and amino acid sequences are presented in sequence listings, adopting an XML format. This international standard, the foundation for US sequence regulations (37 CFR 1.831–1.835), applies to applications with filing dates on or after July 1, 2022.
Key points about the ST.26 sequence listing standard:
- XML Format Requirement: ST.26 mandates the use of a single file in XML 1.0 format, encoded with Unicode UTF-8, adhering to the WIPO Standard ST.26 Document Type Definition (DTD).
- Two Essential Sections: An ST.26 sequence listing in XML comprises a general information section (with bibliographic details) and a sequence data part (housing sequences and their identifying features).
- MPEP Updates: The Manual of Patent Examining Procedure (MPEP) is set to undergo updates addressing the WIPO Standard ST.26.
Purpose and Features of ST.26:
- Standardization Across Offices: ST.26 aims to streamline sequence listing procedures globally, accommodating advancements in biotechnology and aligning with international regulations for sequence databases.
- Global Acceptance: In contrast to the “plain text” TXT format of ST.25, ST.26 mandates a single XML file, fostering global acceptance and compatibility with international databases.
- Benefits for All: The new standard promises benefits for applicants, the public, and examiners by enhancing accuracy and quality in presenting sequences, easing data searches, and facilitating electronic data sharing and database entry for sequence information.
ST.26 brings a wave of efficiency, accuracy, and global compatibility to sequence listings, ushering in a new era for patent applications.
What’s New in ST.26 Sequence Listing Standard?
The ST.26 sequence listing standard brings forth several noteworthy features, revolutionizing the way sequence listings are presented including:
- XML Format Requirement: Sequence listings must now be shared in XML format, departing from the TXT or PDF formats used under ST.25.
- Improved Sequence Depiction: The structured nature of XML format enhances sequence depiction, enabling automation of data verification and streamlining IPO procedures.
- Simplified Applicant and Inventor Information: A single applicant name and inventor name suffices for general information, streamlining the submission process.
- Priority Information Streamlining: Only the oldest priority is necessary, and multiple invention titles in different languages are now permissible.
- Enhanced Sequence Identification: Sequences must be uniquely designated as DNA, RNA, or AA, with mandatory qualifications for accurate molecule identification.
- Expanded Sequence Scope: Unlike ST.25, which only required L-amino acids, ST.26 mandates the inclusion of D-amino acids, nucleotides, branched sequences, and analogs.
- Global Adoption: A single sequence listing can now be adopted globally, eliminating the need for language translations.
- Specified Sequence Disclosures: ST.26 specifies which sequence disclosures must be included, allowed, and how they should be represented.
- Exclusion of Small Sequences: Small sequences are prohibited under the ST.26 standard, ensuring comprehensive sequence representation.
These enhancements in the ST.26 standard signify a significant leap forward in standardization and comprehensiveness, paving the way for more efficient and accurate sequence listings.
ST.25 vs. ST.26: Key Differences
| Category | ST.25 Standard | ST.26 Standard |
| Format | ASCII text format. | UTF-8 (Unicode)-encoded XML format. |
| Sequence Content | Optional: D-amino acids, Linear sections of branched sequences, Nucleotide analogs | Mandatory: D-amino acids, Linear sections of branched sequences, Nucleotide analogs |
| Sequence Annotation | Only feature keys | Key features and qualifiers |
| Sequence Length | Sequences allowed: < 10 nucleotides, < 4 amino acids | Unacceptable: < 10 nucleotides, < 4 amino acids (except “n” and “X”) |
| Priority Applications | Incorporate all priority applications | Only the most important application allowed |
| Applicant and Inventor Names | Names of all applicants and inventors listed | Only one applicant and potentially one inventor name |
| Invention Title | One invention title | Multiple titles in different languages |
| Character Usage | Latin characters for names | Unicode characters, including Latin translation |
| Sequence Classification | Sequences categorized as DNA, RNA, or PRT | Further classification as DNA, RNA, or AA with mandatory “mol type” |
| Organism Names | Organism names: Latin genus/species, Virus, “Artificial sequence,” “Unknown” | Organism names: Latin genus/species, Virus, “Synthetic construct,” “Unidentified” |
| Nucleotide Symbol | “u” for uracil in nucleotide sequences | “u” not recognized; “t” for thymine/uracil; “t” in ST.26 for modified bases |
| Amino Acid Representation | Three-letter acronyms for amino acids | One-letter acronyms for amino acids |
| Variable Residues | Definition needed for “n” and “Xaa” variable residues | Default assumed; definition required for non-default values |
| Feature Location Format | Undefined format for feature locations | Strict formats with symbols allowed in all sequence types |
| Mixed Mode Sequences | “Mixed mode” sequences acceptable | Not permitted; “translation” qualifier for nucleotide translation |
ST.26 introduces these changes to enhance clarity, standardization, and efficiency in the patent application process.
Key Features of the ST.26 Sequence Listing Standard
The ST.26 standard introduces several significant features applicable to all sequences, including nucleotide and amino acid sequences:
- Unique Sequence Identification: Each sequence must possess a distinct sequence identification number. This number should commence at one and increment sequentially by integers. In cases where a sequence is intentionally omitted, a triple zero is used as a placeholder.
- Structured Sections: The sequence listing comprises two primary sections: the general information section and the sequence data section. The document is presented as a single XML file, with the general information section containing bibliographic data linking the sequence listing to its corresponding patent application.
- Sequence Data Elements: The sequence data section contains one or more sequence data elements, each providing detailed information about a single sequence.
- Compliance with Specifications: Adherence to the INSDC (International Nucleotide Sequence Database Collaboration) and UniProt specifications is mandatory when crafting the sequence data elements.
To facilitate compliance with the ST.26 standard, patent applicants can utilize various tools, with the WIPO Sequence Suite emerging as one of the most popular options. Let’s delve deeper into the functionalities and benefits of this software.
Implementing ST.26 Standard with WIPO Sequence Listing Software
Creating sequence listings compliant with the WIPO ST.26 standard is crucial yet complex. While various XML editing tools can be used, experts recommend specialized software like WIPO Sequence Suite. Developed in collaboration with patent offices globally, this software simplifies the creation, verification, and authoring of ST.26-compliant listings.
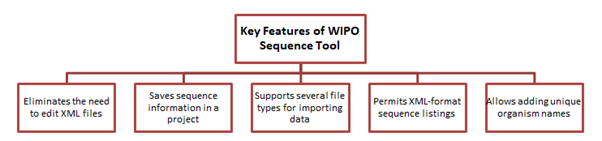
Features of WIPO Sequence Suite:
- Project Management: Easily save and manage project-specific data for seamless workflow.
- Data Input: Import data from various sources including ST.25 listings, facilitating transition to ST.26.
- User-Friendly Interface: Effortlessly add features and qualifiers, verify data, and generate compliant XML listings.
- Validation: Ensure accuracy and compliance with ST.26 standards through built-in validation tools.
WIPO Sequence Validator complements the Suite, providing online validation services for intellectual property offices.
Considerations When Converting ST.25 Listings:
- Data Integrity: Ensure imported ST.25 listings adhere to requirements to avoid complications.
- Project Creation: Importing generates an ST.26 project; additional data is needed for a genuine XML listing.
- Data Analysis: Review detailed Import Reports to understand modifications and prevent data loss risks.
In summary, WIPO Sequence Suite simplifies adherence to ST.26 standards, ensuring accurate and compliant sequence listings for patent applications.
New Inclusion Criteria in ST.26 Sequence Listing Standard
ST.26 ushers in fresh inclusion guidelines absent in ST.25. It mandates unconventional sequence types like D-amino acids, nucleotide analogs, and branched sequences.
Key changes include:
- Minimum Length Requirement: Sequences must be unbranched or linear sections of branched sequences with:
- 10 or more defined nucleotides, or
- 4 or more defined amino acids.
- Exclusion of Short Sequences: Sequences below the threshold must be detailed elsewhere in the patent specification, not within the sequence listing, contrasting ST.25.
- Definition of “Specifically Defined” Residues: Only nucleotides and amino acids explicitly defined (excluding “n” and “X”) count toward length.
- Symbols for Alternative Nucleotides: Annex I of ST.26 provides symbols for alternative nucleotide combinations like “v” for “a or c or g; not t/u.”
These ST.26 changes bring clarity and specificity to sequence listings, offering benefits explored in the next section.
Advantages of ST.26 Sequence Listing Standard
The adoption of the new sequence listing standard, ST.26, brings several advantages, including:
- Streamlined Data Verification: Enhanced automation improves data verification accuracy.
- Simplified IPO Procedures: ST.26 simplifies procedures for intellectual property offices.
- Clearer Sequence Presentation: XML formatting provides a structured layout for clearer sequence presentation.
- Unified Listing: Applicants can provide a single sequence listing for regional, national, or international patent applications.
- Enhanced Precision: ST.26 raises the precision and standard of sequence presentations.
- Ease of Data Searching: Improved sequence data searching capabilities facilitate efficient research and analysis.
- Electronic Data Sharing: Enables seamless electronic sharing and integration of sequence data into digital databases.
- Consensus among IP Offices: ST.26 ensures agreement and consistency among intellectual property offices.
- Accelerated Processing: Increases the speed of intellectual property offices’ processing and enhances data validation automation.
- Improved Submission Quality: The XML format of sequence listings leads to higher submission quality and accuracy.
These advantages underscore the significant improvements brought about by the ST.26 sequence listing standard, benefiting both applicants and intellectual property offices alike.
Best Practices for Smooth Transition into ST.26 Standard
Transitioning into ST.26 sequence listings requires careful consideration and adherence to best practices:
- Know Effective Dates: While ST.25 remains valid for PCT applications submitted before July 1, 2022, all new applications, including those claiming priority, must comply with ST.26. Ensure compliance based on application submission dates.
- Conversion Guidelines: Convert ST.25 listings to ST.26 format for new applications. Ensure compliance with specific requirements outlined by patent offices, such as statements confirming no addition of subject matter beyond the parent application.
- Time Considerations: Allocate sufficient time for creating or converting listings, especially for sequences affected by revisions. Examples include sequences with D-amino acids, nucleotide analogs, thymine-containing RNA or DNA, branched sequences, and additional feature annotations.
- Exercise Caution: When converting ST.25 listings to ST.26 format for applications claiming earlier priority dates or European divisional applications, avoid inadvertently adding or removing subject matter. Take particular caution to comply with strict guidelines from patent offices like the EPO regarding new subject matter and priority entitlement.
By following these best practices, applicants can ensure a seamless transition to the ST.26 standard while maintaining compliance with patent office requirements and avoiding potential issues related to subject matter and priority entitlement.
Why Choose Us: The Sequence Listing Company
Our patent sequence listing services comply with PTO regulations, supporting law firms and corporations with expert draftsmen handling thousands of sequences daily. Order now to ensure accurate IP filings and avoid unnecessary Office Actions.
With years of experience, we offer quick, cost-effective, and accurate sequence listing services. Our draftsmen ensure 100% compliance with patent office rules, equipped with the latest software and expertise in global patent office guidelines. Enjoy a user-friendly platform and prioritize your patent sequence listing needs with us.
- Expertise: Biotech professionals ensure compliance with sequence listing standards.
- Client Satisfaction: We offer free iterations until you’re fully satisfied.
- Efficiency: Benefit from cost-effective solutions with swift turnaround times.
- Compliance: Our services adhere to PTO regulations.
- Expedited Delivery: Receive priority notice from the PTO for urgent projects.
Our Success Stories
Sagacious IP has a team of experts who can create flawless sequence listings for your patent applications. In the past few years, we have helped numerous clients prepare flawless sequence listings and obtain IP protection. Here are some such case studies.
CASE STUDY 1: Empowering Biotech IP Filings
Client: A US-based reputed IP Law Firm
Challenge: The client needed to efficiently handle a large volume of sequence listings for biotech clients’ patent applications while ensuring compliance with complex ST.26 standards and maintaining listing quality.
Solution and Impact:
- Algorithm Development: Sagacious developed custom algorithms for ST26-compliant sequence listings.
- Cost and Time Savings: Sagacious’s expertise reduced resource and time requirements, resulting in significant cost savings.
- Quality Assurance: Rigorous checks ensured high accuracy, delivering over 98% quality listings.
- Achievements: Sagacious delivered 100 listings in 7 months, including complex listings with over 40,000 sequences.
CASE STUDY 2: Streamlining Sequence Listing Preparation for a Top-Tier Law Firm
Client: Major law firm with 1000 attorneys globally
Challenge:
- Handling 150+ sequence listings annually for diverse clients, a resource-intensive task.
- Ensuring compliance with complex ST.26 standards and maintaining listing quality.
- Meeting client demands efficiently while keeping up with biotech advancements.
Solution and Impact:
- Sagacious developed proprietary algorithms for efficient sequence listings.
- Reduced time and resources, providing substantial cost savings.
- Rigorous 3-level checks ensured 98%+ accuracy.
- Delivered 100 high-quality listings in 7 months, including 40,000+ sequence listings.
CASE STUDY 3: Streamlining Sequence Listing Preparations for a Mid-Size Law Firm
Client: A mid-size IP law firm from the UK.
Client’s Situation: The firm faced bandwidth issues, struggling to meet a big client’s demands for high-quality sequence listings due to limited resources. This risked losing the client. The challenge was to find a solution to streamline operations, meet client demands, and ensure compliance.
Challenges:
- Limited Resources and Staff: Scarce resources hindered timely and quality sequence listing preparation.
- Quick Turnaround Requirement: The big client’s urgent needs left little time for other work, demanding a prioritization strategy and efficient processes.
Solution and Impact:
Sagacious provided a dedicated biotech professional as Full Time Engagement, deeply understanding client needs and optimizing efficiency. This ensured quality delivery of sequence listings within strict timelines, meeting client expectations effectively.
These case studies demonstrate our ability to streamline biotech IP filings, ensuring efficiency, compliance, and high-quality outcomes for the client.
Conclusion
With the introduction of WIPO’s ST.26 sequence listing standard, all new patent applications incorporating sequence listings must adhere to these enhanced guidelines. ST.26, a notable improvement over ST.25, imposes additional requirements that applicants need to diligently fulfill. Despite the visual differences between XML and TXT formats, the WIPO Sequence Program provides a user-friendly solution for creating ST.26-compliant sequence listings and offers a clearer representation.
Applicants and agents are encouraged to leverage the WIPO Sequence Suite, which automatically aligns with many of the new ST.26 standards. Acquiring familiarity with this tool ensures a smoother transition and compliance with the latest sequence listing guidelines. For those seeking professional assistance, The Sequence Listing Company’s experts stand ready to create error-free, ST.26-compliant sequence listings efficiently and affordably. Our experienced team guarantees reliable and comprehensive sequence listing services. To know more about our Sequence Listing Service click here.
