All patent applications specifying nucleotide or amino acid sequences must include a sequence listing. The World Intellectual Property Organization (WIPO) has set forth international standards for preparing sequence listings, and failure to comply with these guidelines may result in the rejection of the patent application. WIPO recently introduced the latest sequence listing standard, ST26, which aims to enhance data searching and transfer for patent offices, inventors, and applicants alike.
This article explores ST26 and delves into its features, benefits, drawbacks of the previous standard (ST25), and more.
Limitations of the ST.25 Sequence Listing Standard
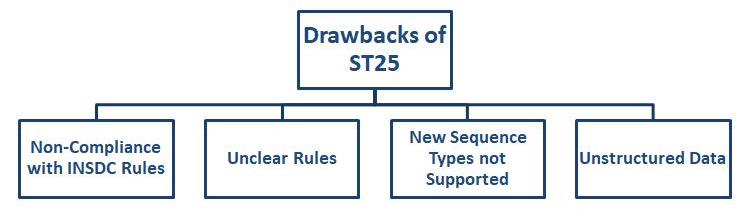
Here are some key concerns with the ST.25 standard that led to the introduction of ST.26:
- ST25 format did not adhere to the INSDC (International Nucleotide Sequence Database Collaboration) requirements, and data was lost when imported into public databases.
- Furthermore, several ST.25 regulations lacked clarity, causing IPOs everywhere to apply and interpret them differently.
- The ST25 format proved difficult to use for automated data exchange and validation since the data was unstructured.
- Another problem with the ST.25 standard was that it did not address the kinds of sequences that are common and used today, such as branching sequences, D-amino acids, and nucleotide analogs. This rendered these sequences unsearchable in databases. Additionally, since the data was unstructured, it was impossible to verify it.
The ST26 sequence listing standard was created due to ST25’s flaws. The primary goal behind the introduction of ST26 is to improve and standardize the filing criteria for sequence listings while also streamlining the process of searching nucleotide and amino acid sequences connected to patent applications.
Changes Introduced by ST.26 Sequence Listing Standard
Through ST.26, WIPO aims to improve the standardization and compatibility of sequence listings. To achieve this goal, a number of modifications have been made, such as:
- .xml file types are required to prevent data loss and support both human and machine-readable data exchange.
- Sequence annotation options, and organism names and options have been updated.
- Three-letter codes are now preferred instead of single-letter codes.
- Modifications have been made to the feature location format.
- Mixed-mode sequences are no longer allowed.
- Only the latest priority information can be included instead of all.
- Language requisitions have been simplified.
- Key differences between the old and new sequence listing standards can be highlighted to enhance understanding.
Now, let us delve into the benefits introduced by the latest sequence listing standard.
Benefits of ST.26 Sequence Listing Standard
- Improved Compatibility: One of the most significant changes introduced by the introduction of ST26 standard is improved compatibility with other software tools. Thus, the new sequence listing standard makes it easier to exchange data between different systems. This improvement will streamline sequence listing preparation, reducing errors and increasing efficiency.
- Enhanced Security: Another key update to the ST.26 standard is the addition of enhanced security measures. WIPO has implemented a new checksum system to ensure that data is transferred securely between different systems. This system helps in preventing data loss or corruption during the transfer process, ensuring that the sequence listing data is accurate and complete.
- More Detailed Descriptions: The new ST.26 standard also provides more detailed descriptions of genetic sequences. The updated format includes additional information, such as annotations and translations, making it easier for patent examiners to understand the invention’s genetic makeup. This change is also aimed at improving the clarity and accuracy of the patent application, increasing the chances of successful patent registration.
- Better Accessibility: Finally, the new ST.26 standard has improved accessibility for users. The format is now available in multiple languages, making it easier for international patent applicants to use the standard. Additionally, WIPO has released a comprehensive user manual that explains the format and its implementation, making it easier for patent applicants to understand and navigate the new standard.
Also Read:- Sequence Listing Errors – You Must Avoid
Major Features of ST.26 Sequence Listing Standard
The key features of the ST.26 Standard include:
- Each sequence must have a unique sequence identification number, with the option to use a triple zero to represent a deliberately skipped sequence.
- The sequence listing is composed of two sections: the general information section and the sequence data section.
- The general information section provides bibliographic data that is used to link the sequence listing with the relevant patent application.
- The sequence data section contains one or more sequence data elements, each providing information on a single sequence.
- The INSDC and UniProt specifications must be followed when creating the sequence data elements.
- A document-type declaration is required to present the sequence listing as a single XML file.
- Other features include requirements for .xml file type to prevent data loss and support both human and machine-readable data exchange, updates to sequence annotation options, and organism names and options, a preference for three-letter codes over single-letter codes, modifications to the feature location format, and simplification of language requisitions.
- Mixed-mode sequences are no longer allowed, and the latest priority information can be included rather than all.
Why Choose The Sequence Listing Company (TSLC) for your Sequence Listing Needs?
Our experts can create ST.26-compliant sequence listings adhering to national and international IP office guidelines.
- We have a team of biotech and bioscience experts with extensive experience in creating compliant sequence listings.
- We use in-house algorithms and specialized software, such as WIPO Sequence Suite, PatentIn 3.5, and BiSSAP, to generate error-free results for our clients.
- We also provide error-free, PTO-compliant sequence listings with fast, cost-effective services with a quick turnaround time.
- Till date, we have prepared over 5000 accurate sequence listings in a format acceptable to PTO.
Conclusion
The ST.26 sequence listing standard is an essential tool for patent applicants in the biotechnology and pharmaceutical industries. With the recent updates from WIPO, the format has become more user-friendly, secure, and compatible with other software tools. These changes will streamline the patent application process, making it easier for inventors to protect their innovations and bring new products to market.
Non-compliance with WIPO’s sequence listing standards may lead to patent application rejection. Let TSLC’s team of patent experts assist you in preparing sequence listings in accordance with the most recent guidelines. Visit our sequence listing service page for further information.
