The World Intellectual Property Organization (WIPO) has introduced a new sequence listing standard, ST.26, which provides a uniform format for submitting nucleotide and/or amino acid sequence listings in patent applications. This new ST.26 sequence listing standard replaces the previous ST.25 format and aims to improve the accuracy and consistency of sequence listings, while also making it easier for patent offices and the public to access and use the sequence data.
In this article, we will explore everything you need to know about the new WIPO ST.26 sequence listing standard, including its features, benefits, how to comply with the standard when submitting sequence listings in patent applications, and much more.
What are Sequence Listings?
Patent specifications in biotech patent applications must provide a thorough explanation of gene mutations, sequencing, regulation, expression, or silencing. This is crucial for patent examiners to fully comprehend the innovation and compare it to prior art. The inclusion of DNA and protein sequences in patent specifications is mandatory as per the guidelines established by patent and trademark offices (PTOs). However, to be effective, these sequences must have a defined structure to ease comprehension.
Sequence listing is the process of converting raw sequences into the necessary format in accordance with the rules of the patent office. A gene is made up of a chain of polynucleotides composed of amino acid codes, such as A, T/U, C, and G, which represent the building blocks of proteins. A unique one-letter code is used to represent each amino acid, and a sequence listing uses “SEQ ID NO.” (for example, SEQ ID NO: 1) to identify the gene or protein and provide the whole sequence.
In case a patent application mentions nucleotide and/or amino acid sequences, the applicant must submit a sequence listing to the relevant intellectual property office (IPO) to ensure that the sequence data is searchable by IPOs and the public. A number of patent offices, such as the European Patent Office (EPO), and United States Patent and Trademark Office (USPTO), share sequence data through publicly available databases, such as EMBL (European Molecular Biology Laboratory) and NCBI (National Center for Biotechnology Information). An international standard for sequence listing can help ensure hassle-free transfers and searching of data on and between such databases.
Sequence listings address a critical problem that patent offices and inventors often face when dealing with biotechnology innovations. We deep dive into this in our next section.
What is the Need for Sequence Listings?
A sequence listing is a document that compiles all the biological sequence information provided in a patent application into a standardized format. It allows for the recording and sharing of biological sequence data from patent applications to searchable databases utilized by national and public patent offices worldwide. To ensure the standardization of this document, it is essential to include a sequence listing that meets the requirements outlined by international organizations, regardless of the sequence’s origin.
Biological sequences are significant references for future studies and inventions. The sequence listing presents all the biological sequence information disclosed in a patent application in one document, including the nucleotide (RNA or DNA) and/or amino acid protein sequences.
Previously, all sequence listings in patent applications had to comply with the WIPO’s ST.25 sequence listing standard. Moreover, applicants used software, such as PatentIn and BiSSAP, to prepare ST.25-compliant sequence listings and delivered the same to the IPOs on paper or in .txt format. However, a new sequence listing standard, ST.26, has been established, and all sequence listings must now adhere to these new guidelines. Failure to meet WIPO’s sequence listing standards can result in the rejection of a patent application.
It is crucial to understand the previous sequence listing norms before familiarizing oneself with the new standard. The next section of this article will provide insight into the previous sequence listing requirements.
About ST.25 Sequence Listing Standard
The WIPO’s ST.25 standard, which was adopted in 2009, provides guidelines for presenting nucleotide and amino acid sequences in a sequence listing. The US sequence regulations (37 CFR 1.821–1.825) were based on this standard. ST.25 format is mandatory for any patent applications filed before July 1, 2022, that require a sequence listing.
The “header” fields, marked from 110 to 170, are included only once in the beginning of the sequence listing and apply to all sequences included in it. Since sequence listings are often submitted separately from the patent application, these header fields are used to link the sequence data to the corresponding patent application.
Each sequence in an ST.25 sequence listing is assigned a unique numbered identifier, starting from “1” and incrementing sequentially. The genetic identification of each sequence can be found in the <210> field and the first line of the <400> field. Sequences mentioned in the patent application, including in the description, claims, or drawings, are identified by their respective sequence identifiers, which are preceded by the prefix “SEQ ID NO:” from the sequence listing. The WIPO ST.25 standard was the norm for sequence listings until the introduction of ST.26 sequence listing standard in July 2022.
Shortcomings of ST.25 Sequence Listing Standard
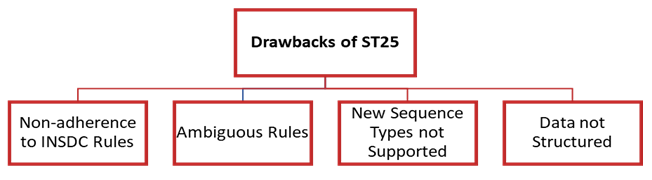
The ST.25 standard had several limitations that called for the introduction of ST.26. These include the following:
- The format specified by ST.25 did not adhere to International Nucleotide Sequence Database Collaboration (INSDC) specifications, resulting in data loss when transferred to public databases.
- Some of the regulations outlined in the ST.25 standard lacked clarity, leading to inconsistent interpretations and applications across IPOs worldwide.
- The unstructured data format of the ST.25 standard also posed challenges for automated validation and data exchange.
- ST.25 did not cover popular and widely used sequence types, such as nucleotide analogs, D-amino acids, and branched sequences, which made it difficult to locate these sequences in searchable databases. Furthermore, the unstructured nature of the data made it challenging to verify the information provided.
The limitations of the ST.25 standard led to the creation and introduction of the ST.26 sequence listing standard. The main aim of the updated guidelines is to establish consistent and improved requirements for sequence listings, while also simplifying the process of searching for nucleotide and amino acid sequences associated with a patent application. The following section provides a detailed overview of the ST.26 sequence listing standard.
About ST.26 Sequence Listing Standard
The ST.26 is an international standard that specifies the format for presenting nucleotide and amino acid sequences in a sequence listing using an XML format. It was adopted in October 2021 and became effective on July 1, 2022. The WIPO ST.26 sequence listing standard serves as the foundation for the US sequence regulations (37 CFR 1.831–1.835). The standard mandates that sequence listings for all applications filed on or after July 1, 2022, be presented in the XML format in a single file encoded with Unicode UTF-8 and adhering to the WIPO Standard ST.26 Document Type Definition (DTD).
The ST.26 sequence listing standard is structured in XML format, and it comprises of two primary sections. These sections are explained in detail below:
- The first section is the general information part that includes essential bibliographic data, such as the application number, filing date, applicant and inventor’s names, the invention’s title, and the earliest priority application.
- The second section is the sequence data part, which contains one or more sequences, along with all the features that identify those sequences.
The MPEP (Manual of Patent Examining Procedure) updates related to WIPO Standard ST.26 are currently in development and will be published soon.
The ST.26 standard aims to establish uniform procedures for sequence listing filing requirements that are consistent with advancements in biotechnology and comply with international regulations for sequence databases. Its objective is to create a globally accepted sequence listing format that is compatible with other international databases and adheres to INSDC specifications.
Unlike the “plain text” TXT format of ST.25, the ST.26 sequence listing standard requires that sequence listings be presented in a single XML file. This adoption of XML format is intended to facilitate data searching, particularly in public databases.
The ST.26 standard introduces several key features, including:
- Applicants can now file a single sequence listing that can be used for both international, national and regional procedures.
- Improvements in the accuracy and quality of presented sequences will benefit the public, examiners, and applicants alike.
- More searchability of sequence listings, and hassle-free electronic data sharing and database entry for sequence information.
What’s New in ST.26 Standard?
The ST.26 sequence listing standard has brought about several changes to the way sequence listings are submitted with patent applications, including:
- Sharing of sequence listings in XML format instead of TXT or PDF, which enhances the depiction of sequences and facilitates data verification and IPO procedures.
- The submission of a single applicant name and inventor name for the general information contained in the sequence listing, which is a formal modification rather than a substantive one.
- The requirement to submit only the oldest priority information and the ability to submit multiple titles of the invention, each in a different language.
- Sequences to be designated as DNA, RNA, or AA, with a mandatory qualification to identify the molecule more accurately.
- The inclusion of D-amino acids, nucleotides, branched sequences, and analogs, in contrast to the ST.25 sequence listing rules that only required L-amino acids.
- The ability to adopt a single sequence listing across the globe, without the need for language translations.
- Sequence disclosures allowed, and how these sequences must be represented are now clearly specified.
- The prohibition of small sequences under the ST.26 standard.
Key Features of ST.26 Sequence Listing Standard
- Each sequence must have a unique identification number, starting at one and increasing sequentially by integers.
- A triple zero should be used to represent a deliberately skipped sequence.
- The sequence listing must be provided as a single XML file with a document-type declaration.
- The sequence listing consists of two sections: the general information section and the sequence data section.
- The general information section includes bibliographic data used to associate the sequence listing with the relevant patent application.
- The sequence data section contains one or more sequence data elements, each providing information about a single sequence.
- The sequence data elements must adhere to the INSDC and UniProt specifications.
Also Read – The Art of ST.26 Sequence Listing Preparation: Best Practices for Accuracy and Compliance
Putting ST.26 Sequence Listing Standard into Practice with Relevant Tools
Experts recommend using specialized software, such as the WIPO Sequence Suite, to construct a sequence listing in compliance with the complex WIPO ST.26 sequence listing standard. This tool was created by WIPO in partnership with patent offices worldwide to simplify the process of creating, verifying, and authoring ST.26-compliant sequence listings for all users.
The WIPO Sequence Suite provides a user-friendly interface that simplifies the creation of ST.26-compliant XML format sequence listings. The tool enables users to input data from various file formats, including ST.25 sequence listings, ST.26 sequence listings projects, and multi-sequence format files, among others. Alternatively, users can prepare a sequence listing by entering a name and adding the sequences. In addition, the software can validate sequence listings in XML format, ensuring compliance with relevant standards. Overall, the WIPO sequence tool is an efficient solution that enables applicants to create accurate and compliant sequence listings with ease. The WIPO sequence listing tool allows one to:
- The WIPO Sequence Suite offers two options: “import project” and “import sequence listings,” which allow users to upload additional files and continue working on them. Additionally, all previous projects can be accessed easily under the project header and continue working on them.
- The sequence listing verification feature is another useful tool that allows users to evaluate their sequence listings. This feature helps to identify and store application and sequence data specific to the project.
- Furthermore, users can choose from a set of simple drop-down options to add feature keys and qualifiers to sequences.
- WIPO’s tool also allows users to verify project data and produce sequence listings in XML format that is compliant with the latest guidelines.
- The accuracy of an existing XML format sequence listing can be confirmed, and project data can be created in “human-readable” format for easy review.
- Additional features include the ability to save unique applicant and inventor details for projects, record unique organism names.
- It can also import ST.25 sequence listings, ST.26 sequence listings, ST.26 projects, raw files, multi-sequence format, and FASTA files for processing.
- Lastly, the tool also eases the translation of free text qualifier values, making it a versatile and user-friendly option for managing sequence data.
The WIPO Sequence Validator is an online tool designed to assist intellectual property offices in verifying the compliance of submitted sequence listings with the WIPO ST.26 standard. This software was developed through a collaboration between the Committee on WIPO Standards and patent offices worldwide. Its primary purpose is to offer a web service for validating XML files in WIPO ST.26 format, ensuring adherence to the established sequence listing standard.
This tool reads files from a local file system and produces a report that contains the outcomes of the validation process. Additionally, the tool can send the results of the verification process, which includes the verification report, to an endpoint as per requirement.
It is important to bear in mind the following considerations when converting an ST.25 sequence listing to an ST.26 project:
- It is essential to ensure that the ST.25 sequence listing imported into the WIPO sequence tool is legitimate and complies with all ST.25 requirements. Otherwise, there is a risk of data loss and unforeseen complications that may arise due to importing an erroneous ST.25 sequence listing.
- Importing an ST.25 sequence listing results in the creation of an ST.26 project, rather than an ST.26 XML format sequence listing. The reason for this is that an ST.25 sequence listing does not include mandatory elements such as the “mol type” qualifier that are present in an ST.26 sequence listing. To generate a genuine ST.26 XML sequence listing, additional data needs to be added to the resulting ST.26 project.
- It is highly important for users to thoroughly examine every note listed in the comprehensive “Import Report” generated by the WIPO sequence tool after importing ST.25 text files. This is necessary to understand the modifications made to the data and to take appropriate measures to avoid any potential loss of data.
Recommended Practices for Smooth Transition into ST.26 Standard
Follow these practices to ensure a smooth transition into the ST.26 sequence listing standard:
- The ST.25 standard will continue to be applicable for PCT applications that are filed before July 1, 2022 and later enter the European or UK national phase. However, all new applications, including European divisional applications filed after July 1, 2022 and applications claiming priority from previously filed applications, must adhere to the ST.26 standard.
- The European Patent Office (EPO) demands the conversion of ST.25 sequence listings to ST.26 format and a statement stating that the new sequence listing does not include any additional subject matter beyond the parent application to file a divisional patent application submitted on or after July 1, 2022. This is specific to applications that descend from a patent application filed before the mentioned date. In contrast, the UK Intellectual Property Office (UKIPO) mandates that sequence listings accompanying new divisional applications filed in Great Britain must be provided in the same format as the parent application.
- When applicants are creating new ST.26-compliant listings or converting existing ST.25 listings to the ST.26 format, it is important to keep in mind that additional time may be required, especially for listings that contain sequences affected by revisions, such as
- Sequences with nucleotide analogs or D-amino acids
- DNA or RNA containing the nucleotide thymine
- Branched sequences
- Sequences with additional feature annotations
- When preparing ST.26-compliant sequence listings for applications that claim an earlier priority date or European divisional applications, it is crucial to exercise great care to ensure that no new subject matter is added or any existing subject matter is removed. This is particularly important due to the EPO’s strict policies regarding new subject matter and priority entitlement. Any inadvertent addition or removal of subject matter in the converted ST.26 sequence listing could pose a significant problem if filed with the EPO.
Why Choose The Sequence Listing Company (TSLC) for your Biotech Patent Applications?
The Sequence Listing Company is a highly reputable and globally recognized intellectual property service provider. Our team of patent professionals has the expertise to create sequence listings that meet the latest guidelines of national and international IP offices. With our exceptional capabilities, The Sequence Listing Company is the perfect choice for organizations seeking a reliable and efficient partner for their sequence listing needs, such as:
- Experts with Rich Experience in Creating Compliant Sequence Listing
Our team comprises experts in biotechnology and biological sciences who boast a comprehensive understanding of the regulatory frameworks of various jurisdictions, including WIPO and the USPTO. Moreover, our experts undergo comprehensive training to ensure that they are capable of crafting listings that comply with the most up-to-date standards.
The team collaborates with the client and furnishes any supplementary details necessary for the preparation of a sequence listing, as mandated by the patent and trademark office. To optimize efficiency and produce client-specific outcomes, we regularly incorporate our proprietary algorithms with sequence listing preparation tools such as WIPO Sequence Suite, PatentIn 3.5, and BiSSAP software.
- Error-free PTO-Compliant Sequence Listings
Over the course of the previous year, The Sequence Listing Company has successfully generated and submitted more than thousands of sequence listings in a format that meets the requirements set by the PTO, and without any errors. Our experts have delivered sequence listing projects of up to 2.5 million sequences. We have also extensively worked on converting existing ST.25 format sequence listings to ST.26-compliant sequence listings.
- Low Cost and Quick Turnaround Time
Our sequence listing services offer efficient turnaround times and affordable pricing. We can help you save big as compared to other sequence listing service providers. Furthermore, we turnaround most projects within three to five working days.
Success Stories
Here are a few success stories that demonstrate our expertise in sequence listing preparation.
Case Study 1: A leading US-based IP law firm sought a reliable partner to handle the significant volume of sequence listings required for its biotech clients’ patent applications. The client wanted a solution that could ensure accuracy, compliance, and timely delivery while reducing costs and streamlining the process of preparing and filing sequence listings. TSLC provided a dedicated resource and set up internal processes to optimize turnaround time and cost. We delivered 20 sequence listings in less than 3 months with a high level of accuracy and precision, providing a significant impact on the client’s business. The success of the project gave the client a competitive advantage in the industry, enabling them to scale up their filings and meet the increasing demand for sequence listings.
Case Study 2: A top-tier IAM 1000 law firm needed a vendor to handle 150+ sequence listings annually for their clients’ patent applications. The law firm was facing challenges in handling a large volume of requests while ensuring compliance with the latest ST.26 standards, maintaining quality, accuracy, and keeping up with the latest advancements in biotechnology and genomics. TSLC developed algorithms in-house to generate sequence listings as per the recent ST.26 standards, reducing the time and resources required, providing significant cost savings to the client. We also ensured the accuracy of their listings through a rigorous 3-level check process, providing high-quality work and further reducing the need for additional resources and time. We delivered 100 sequence listings for the client in just 7 months, including 4 sequence listings with over 40,000 sequences and over 98% quality.
Case Study 3: A US-based biotech law firm needed to convert a sequence listing of 2.5 million sequences into a new ST.26 listing in compliance with US patent law standards. They faced challenges of limited time availability, lack of expertise, handling multiple files, and risk of errors and rejection. TSLC’s team of biotech professionals collaborated with the client’s team to successfully and accurately deliver the listing in ST.26 standards in just three weeks. This helped the client meet their critical deadlines and secure their intellectual property without any complications or delays. The successful partnership between TSLC and the client led to further collaborations in the future.
Conclusion
The WIPO ST.26 sequence listing standard is now mandatory for any new application that involves a sequence listing. To comply with this standard, applicants must ensure that their submissions adhere to the additional guidelines provided. While XML and TXT files have distinct formats, the new WIPO sequence program facilitates the creation of ST.26-compliant sequence listings and offers a more comprehensible presentation of the data. The WIPO Sequence Suite is a useful tool that can help applicants or agents file applications with sequence listings that conform to the new ST.26 standards. Therefore, it is recommended that applicants acquire and familiarize themselves with this program.
The Sequence Listing Company boasts a team of seasoned experts who specialize in crafting precise and error-free sequence listings for both domestic and global patent applications. With years of experience under our belt, we can efficiently produce ST.26-compliant sequence listings at a reasonable cost. Our impressive track record includes successfully completing over thousands of projects for more than 5,000 clients across 100+ nations and 16+ languages. To learn more about our proficiency and accomplishments in this field, please visit our ST.26 sequence listing service page.
